NEWSLETTER
MANUSCRIPT
REGIONAL ANESTHESIA FOR PATIENTS WITH IMPLANTED
PERIPHERAL NERVE STIMULATORS: TO DO OR NOT TO DO
_
ABSTRACT
Peripheral nerve stimulation (PNS) is a relatively novel therapeutic intervention that has been gaining popularity in the management of various chronic pain conditions. The new intervention has shown promising results with a narrow risk margin for complication and has gained US Food and Drug Administration (FDA) approval. Stimulation targets one or two peripheral nerves supplying the target region. Duration of treatment varies from 60 days for temporary microelectrodes to permanent implants that are kept indefinitely. With the increasing number of patients with implanted peripheral nerve stimulators the situation is starting to arise when they present for a surgical procedure that warrants a regional anesthesia block. Basal electric stimulation for neuromodulation of chronic pain is usually not sufficient to ameliorate post-procedural pain. Patients therefore require supplemental peripheral nerve block (PNB) with an in-situ peripheral nerve stimulator sometimes to the same target. In this article, we discuss peripheral nerve stimulators and their implications in the peri-operative settings with emphasis on an adjunctive PNB for acute pain. 6
INTRODUCTION
PNS gradually emerged over the last two decades as a minimally invasive intervention for chronic extremity pain. Multiple studies have demonstrated the potential for clinically significant pain relief and improvements in quality of life, including reductions in disability, decreased analgesic usage, increases in daily activities, and improvements in sleep. This relatively new technique is continuously evolving and has shown success in treating many chronic pain conditions, including nerve injury, complex regional pain syndrome, occipital neuralgia, and post-surgical pain.1 The advent came to build on the legacy of spinal cord stimulation (SCS) that evolved over the second half of the last century from the introduction of gate control theory of neuro-modulation in 1965 and the first commercially available SCS system in 1968 ending with FDA approval in 1977. SCS is now an established treatment in chronic pain practices in North America for intractable chronic pain. Building on the successful integration of SCS in clinical practice and further encouraged by the opioid pandemic, PNS has gained interest as a novel tool in pain management. The potential for PNS to reduce opioid analgesic usage was demonstrated in preclinical studies.2-6 Theories on the analgesic mechanism of PNS all stem from the gate-control theories through which PNS exerts its effect through central neuromodulation of pain pathways or reduce peripheral mediators of pain. 7-9 Despite evidence of its analgesic utility, earlier application of PNS via open neurosurgical placement limited its clinical adoption due to invasiveness and complications. Improvement of the PNS technology led to the modern systems that consist of one or two microelectrodes placed percutaneously under ultrasound guidance similar to regional nerve blocks. Ultrasound guided percutaneous PNS were first reported in 2009 by Huntoon and Burgher for chronic neuropathic pain.10-11 In 2014, Rauck et al reported significant reduction in pain scores in nine out of fourteen patients with phantom limb pain. 12 More interestingly, data from a multicenter, randomized, double-blind, placebo-controlled trial on phantom limb showed a significant reduction in average pain scores up to 12 months after a 60-day PNS treatment. 13 Temporary transcutaneous PNS, for a maximum of 60 days, was approved by the FDA in 2016 for symptomatic relief of chronic, intractable pain, post-surgical and post-traumatic acute pain. The FDA approval paved the way for wider use of PNS across various pathological entities and different clinical scenarios.
PNS For Chronic Pain Conditions
Currently the only FDA approved indication for PNS use is for chronic intractable pain after failure of other less invasive treatment modalities. These chronic pain conditions include post-amputation extremity pain, complex regional pain syndrome, neuropathies, osteoarthritis and chronic headaches. Complex regional pain syndrome (CRPS) and post amputation pain which include residual limb pain 7 (RLP) and phantom limb pain (PLP) are both chronic, extremity pain syndromes known for resilience to most first line pain management strategies hence represented a good target to test the utility of nerve stimulation after exhaustion of medical therapy. Electrical stimulation has been tried in many historical studies on PLP even preceding the currently available PNS systems. 4,14,15 In one review of 117 patients receiving PNS who were followed up to 53 months, 65% reported an increase in their activities of daily living and more than 75% were satisfied with therapy. 16 More recently a multicenter, double blinded, randomized controlled trial investigated the efficacy of percutaneous PNS in PLP and found a significantly greater proportion of subjects receiving PNS (n=7/12, 58%, p=0.037) demonstrate ≥50% reductions in average postamputation pain during weeks 1-4 compared with subjects receiving placebo (n=2/14, 14%). [13] Many case reports on CRPS have been published illustrating the beneficial effect of PNS in patients with reflex sympathetic dystrophy. 17 In a large retrospective series by Chmeila et al, 165 patients had PNS systems implanted for a diagnosis of CRPS. Investigators reported decreased pain scores at 12-months follow up, reduced number of patients on chronic opioid therapy and 51% of patient reporting improvement in functional status over a median follow up of 74 months. 18 Chronic neuropathic pain especially post-traumatic has also been articulated as an indication of PNS by the FDA. Eisenberg et al, applied PNS in 46 suffering from intractable pain due to peripheral nerve injuries where the majority of patients (78%) had long term relief of >50%.19 In a small series by Huntoon et al, 75% of patients with extremity neuropathic pain refractory to other therapies achieved 50% or greater pain relief after a brief period (3-7 days) of stimulation. 11 PNS also emerged as a useful tool in the treatment of intractable headaches and facial pain syndromes by targeting the terminal branches of the cervical plexus most commonly the greater occipital nerve but also supra-orbital and auriculo-temporal nerves. Schwedt et al reported significant improvement in severity and frequency of headaches across 15 patients with medically refractory headaches, an effect that was sustained on the long term with mean follow up 19 months. 20 In a recent study, Zhou et al reported improvement in head pain intensity, duration and frequency in twenty four patient who had permanent PNS implanted after a successful trial. 21
Peri-Operative Considerations
Authors foresee the next arena for PNS will be in the peri-operative settings. Whether primarily as an acute pain management technique, perhaps an adjunct to conventional PNBs. PNS does possess some functional qualities that can support its utilization in the peri-operative settings. Theoretically, PNS does not induce proprioception, motor or sensory deficits as opposed to conventional local nerve blocks which can aid in post-operative ambulation and reduce risk of falls. It also spares the patient systemic effects of opioids and even fear of systemic toxicity with higher doses of local anesthetic 8 nerve catheters. Finally, PNS can be implanted for much longer duration so provide a more sustained analgesic effect. In contrast to regional block catheters, the increased duration of treatment of PNS is associated with extremely low infection rate (1 in 32000 indwelling days). 23
Based on results from Ilfeld’s group, it appears that analgesia is not only possible but it is very effective for a number of ambulatory procedures. Current barriers include insurance coverage in the perioperative setting. Moreover, if designing an anesthetic plan that matches the intensity of a surgical stimulus, peripheral nerve blockade with local anesthetic may be more reliable and accessible. In the future, PNBs with local anesthetic could be combined with PNS for patients who are at risk for uncontrolled postoperative pain. When performing nerve block procedures, staying away from the PNS leads would prevent damage to the indwelling PNS device. The table below summarizes a list of potential indications for combined PNS and nerve block with local anesthetic. (Table 1)
YES: Regional Blocks Are Safe To Perform With PNS Implant In Place
Spinal anesthesia and epidural catheters have been used in select patients with SCS who present for surgical procedures and in laboring women. 23 When considering spinal anesthesia, imaging of the spine gives an insight into the position of the SCS and the feasibility of lumbar puncture. Since there will be epidural fibrosis following SCS, epidural catheter placement for analgesia is usually not recommended and in most cases avoided.
Unlike SCS, PNS electrodes tend to be small (depending on the company of PNS) and percutaneously placed. Thicker, permanent electrodes can be identified by ultrasound imaging and radiological imaging. Thinner 60 day temporary electrodes on the other hand need radiological imaging to ascertain the location. Also, a part of the 60 day temporary electrode is outside the skin giving us an idea where the electrode most likely would be. Further, the amount of scarring with a percutaneous PNS implantation should be less than an open procedure.
Most PNS implants, at this time, are being placed for chronic pain. The acute exacerbation of the pain during and after a surgical procedure necessitates a regional block. This could be achieved through a single injection or a catheter for continuous infusion (Figure 1). Regional anesthesia can be safely performed either distal or proximal to the implanted lead without any untoward effects to the PNS system. (Table 1) With better image quality of ultrasound machines and availability of echogenic needles, the theoretical chance of lead disruption or lead fracture is at the most minimal to none. Also, the electrodes are very malleable and tensile making them resistant to any damage from an accidental 9 needle to electrode contact. A clear communication between the pain physician, anesthesiologist, surgeon, regional team and the patient would ensure a safe and effective way of administering perioperative nerve blocks.
NO: Regional Blocks Are Not Necessary And Have Risk
In patients with existing peripheral nerve stimulators, the addition of PNBs may not be warranted in the perioperative setting. First, the safety profile of combining the two interventions has not been established. Many of these patients already have pre-existing neuropathy, which is a relative contraindication for offering PNBs in some institutions. 24 Peripheral nerve catheters would add another indwelling object inside of the patient. The resulting infection risk is not known. Because the American College of Graduate Medical Education (ACGME) anesthesiology residency training curriculum does not specifically cover the management of peripheral nerve stimulators, anesthesiologists are not expected to know about lead placement or wire location. Without guidelines, anesthesiologists are guessing as to the minimum safe distance to place a PNB to avoid the wires of the indwelling device. Although the risk of error has not been studied in this specific scenario, it is known that increasing complexity of treatment can lead to iatrogenic complications.
Next, there have not been any studies determining the synergistic or antagonistic effect of PNBs when done in conjunction with PNS. The reasons for performing a PNB would include the following: decreased pain scores, decreased opioid-related side effects, decreased anesthetic-related complications, and increased patient satisfaction. It is not yet clear if the addition of a PNB would produce any of these outcomes, especially in cases where general anesthesia is necessary. Overall, PNBs can be done, but it may not be necessary in the current perioperative setting.
REFERENCES
- 1M. Corriveau, W. Lake, and A. Hanna, Nerve Stimulation for Pain, Neurosurg Clin N Am, vol. 30, no. 2, pp. 257-264, Apr 2019
- G. B. Racz, T. Browne, and R. Lewis, Peripheral stimulator implant for treatment of causalgia caused by electrical burns, Tex Med, vol. 84, no. 11, pp. 45-50, Nov 1988.
- D. W. Strege, W. P. Cooney, M. B. Wood, S. J. Johnson, and B. J. Metcalf, Chronic peripheral nerve pain treated with direct electrical nerve stimulation, J Hand Surg Am, vol. 19, no. 6, pp. 931-9, Nov 1994
- D. M. Long, Electrical stimulation for relief of pain from chronic nerve injury, J Neurosurg, vol. 39, no. 6, pp. 718-22, Dec 1973
- W. P. Cooney, Electrical stimulation and the treatment of complex regional pain syndromes of the upper extremity, Hand Clin, vol. 13, no. 3, pp. 519-26, Aug 1997.
- R. J. Mobbs, S. Nair, and P. Blum, Peripheral nerve stimulation for the treatment of chronic pain, J Clin Neurosci, vol. 14, no. 3, pp. 216-21; discussion 222-3, Mar 2007
- J. M. Chung, Z. R. Fang, Y. Hori, K. H. Lee, and W. D. Willis, Prolonged inhibition of primate spinothalamic tract cells by peripheral nerve stimulation, Pain, vol. 19, no. 3, pp. 259-275, Jul 1984
- P. D. Wall and M. Gutnick, Ongoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuroma, Exp Neurol, vol. 43, no. 3, pp. 580-93, Jun 1974
- J. Ellrich and S. Lamp, Peripheral nerve stimulation inhibits nociceptive processing: an
electrophysiological study in healthy volunteers, Neuromodulation, vol. 8, no. 4, pp. 225-32, Oct 2005 - M. A. Huntoon, B. C. Hoelzer, A. H. Burgher, M. F. Hurdle, and E. A. Huntoon, Feasibility of ultrasound-guided percutaneous placement of peripheral nerve stimulation electrodes and anchoring during simulated movement: part two, upper extremity, Reg Anesth Pain Med, vol. 33, no. 6, pp. 558-65, 2008 Nov-Dec 2008
- M. A. Huntoon and A. H. Burgher, Ultrasound-guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: original cases and outcomes, Pain Med, vol. 10, no. 8, pp. 1369-77, Nov 2009
- Rauck RL, Cohen SP, Gilmore CA, North JM, Kapural L, Zang RH, Grill JH, Boggs JW. Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation. 2014 Feb;17(2):188-97.
- C. Gilmore CA, Ilfeld BM, Rosenow JM, Li S, Desai MJ, Hunter CW, Rauck RL, Nader A, Mak J, Cohen SP, Crosby ND, Boggs JW. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2019 Nov 17:rapm-2019-100937 11
- J. N. Campbell and D. M. Long, Peripheral nerve stimulation in the treatment of intractable pain, J Neurosurg, vol. 45, no. 6, pp. 692-9, Dec 1976
- D. M. Long, D. Erickson, J. Campbell, and R. North, Electrical stimulation of the spinal cord and peripheral nerves for pain control. A 10-year experience, Appl Neurophysiol, vol. 44, no. 4, pp. 207-17, 1981
- S. Cohen SP, Gilmore CA, Rauck RL, Lester DD, Trainer RJ, Phan T, Kapural L, North JM, Crosby ND, Boggs JW. Percutaneous Peripheral Nerve Stimulation for the Treatment of Chronic Pain Following Amputation. Mil Med. 2019 Jul 1;184(7-8):e267-e274
- A. V. Fritz, G. Ferreira-Dos-Santos, M. F. Hurdle, and S. Clendenen, Ultrasound-guided Percutaneous Peripheral Nerve Stimulation for the Treatment of Complex Regional Pain Syndrome Type 1 Following a Crush Injury to the Fifth Digit: A Rare Case Report, Cureus, vol. 11, no. 12, p. e6506, Dec 2019
- Chmiela MA, Hendrickson M, Hale J, Liang C, Telefus P, Sagir A, Stanton-Hicks M. Direct Peripheral Nerve Stimulation for the Treatment of Complex Regional Pain Syndrome: A 30-Year Review. Neuromodulation. 2020 Oct 24.
- E. Eisenberg, H. Waisbrod, and H. U. Gerbershagen, Long-term peripheral nerve stimulation for painful nerve injuries, Clin J Pain, vol. 20, no. 3, pp. 143-6, 2004 May-Jun 2004
- T. J. Schwedt, D. W. Dodick, J. Hentz, T. L. Trentman, and R. S. Zimmerman, Occipital nerve stimulation for chronic headache–long-term safety and efficacy, Cephalalgia, vol. 27, no. 2, pp. 153-7, Feb 2007
- Zhou L, Ashkenazi A, Smith JW, Jen N, Deer TR, Zhou C. Long-Term Clinical Outcome of Peripheral Nerve Stimulation for Chronic Headache and Complication Prevention. Anesth Pain Med. 2016 Jul 3;6(4):e35983.
- Ilfeld BM, Gabriel RA, Saulino MF, Chae J, Peckham PH, Grant SA, Gilmore CA, Donohue MC, deBock MG, Wongsarnpigoon A, Boggs JW. Infection Rates of Electrical Leads Used for Percutaneous Neurostimulation of the Peripheral Nervous System. Pain Pract. 2017 Jul;17(6):753-762
- Patel S, Das S, Stedman RB. Urgent cesarean section in a patient with a spinal cord stimulator: implications for surgery and anesthesia. Ochsner J. 2014 Spring;14(1):131-4.
- Abcejo AS, Sviggum HP, Mauermann ML, Hebl JR, Mantilla CB, Hanson AC, Lin Y, Jacob AK. Perioperative Nerve Injury After Peripheral Nerve Block in Patients With Previous Systemic Chemotherapy. Reg Anesth Pain Med. 2016 Nov/Dec;41(6):685-690.
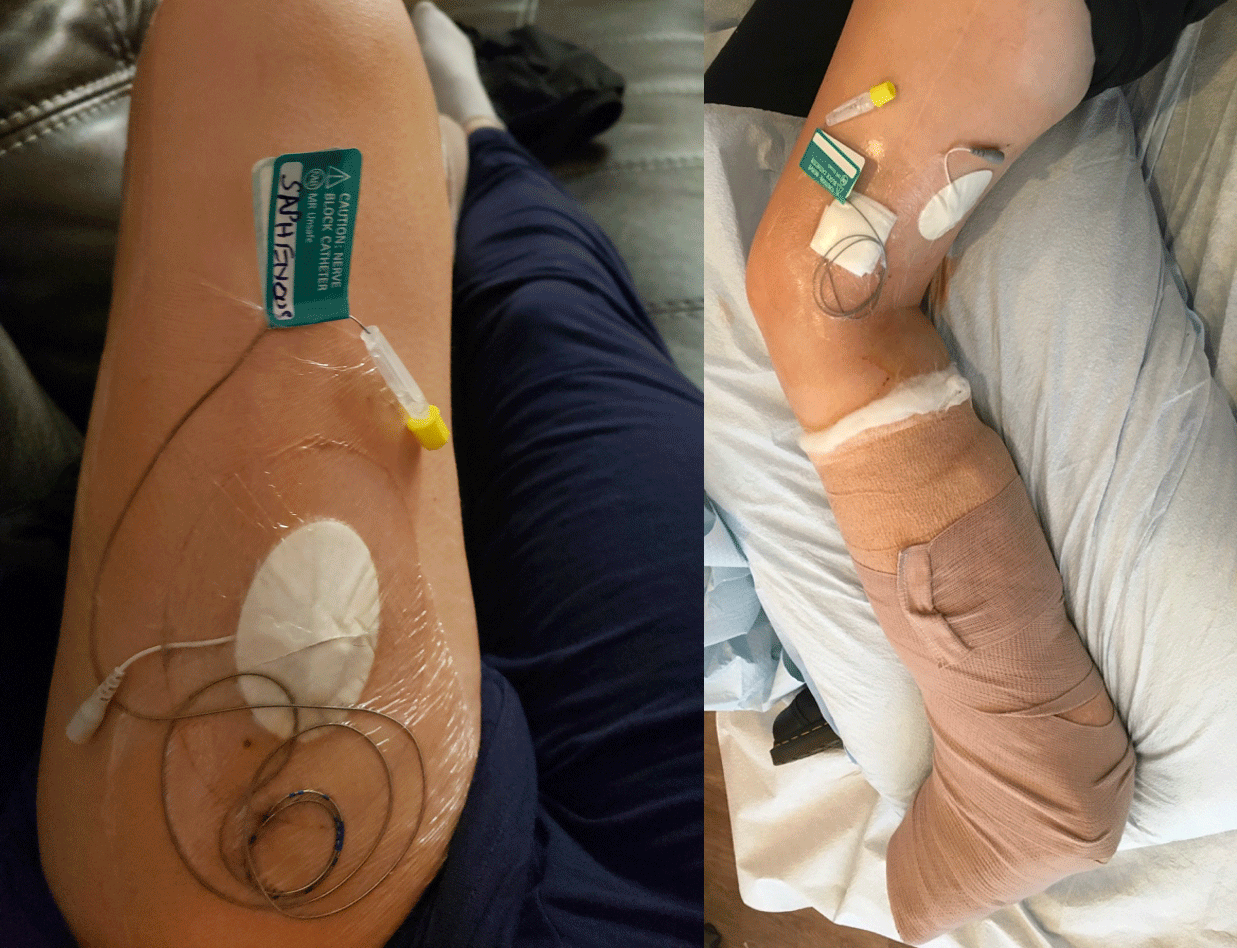
Figure 1: An adductor canal (saphenous nerve) catheter and sciatic nerve catheter in a patient with saphenous and sciatic 60 day PNS implant to manage acute on chronic postoperative pain following total ankle replacement
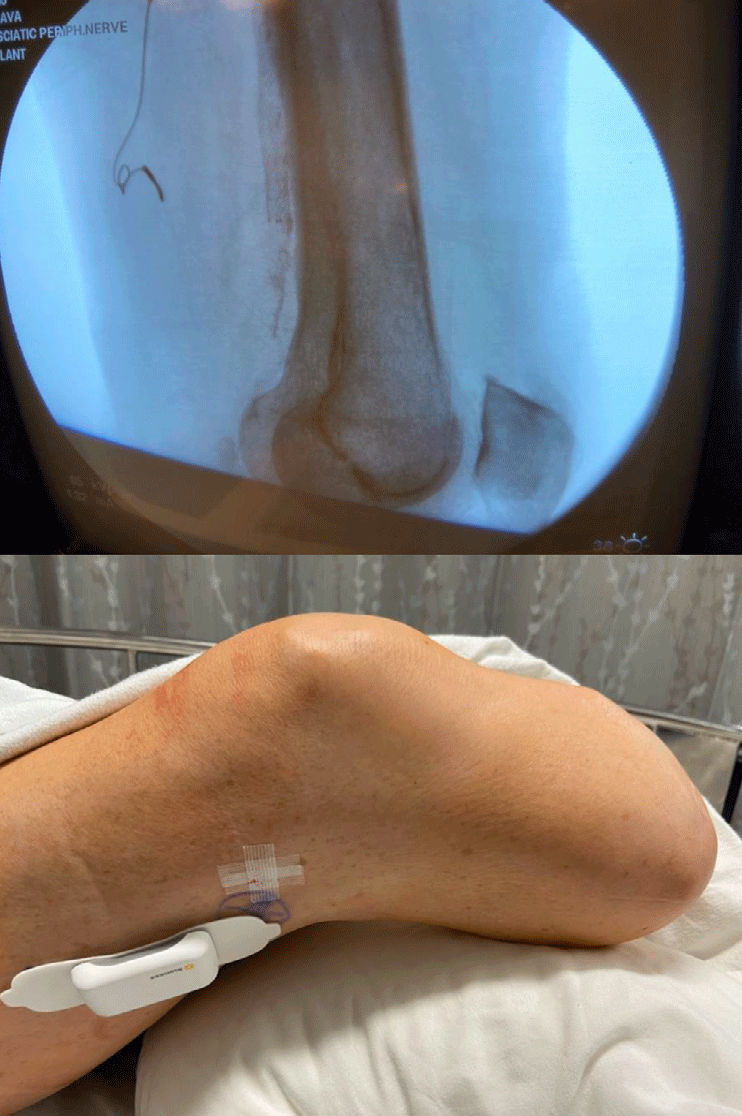
Figure 2: Sciatic permanent PNS implantation in a patient with post amputation stump pain. Post procedure X-ray with PNS electrode and post procedure external pulse transmitter along the lateral thigh
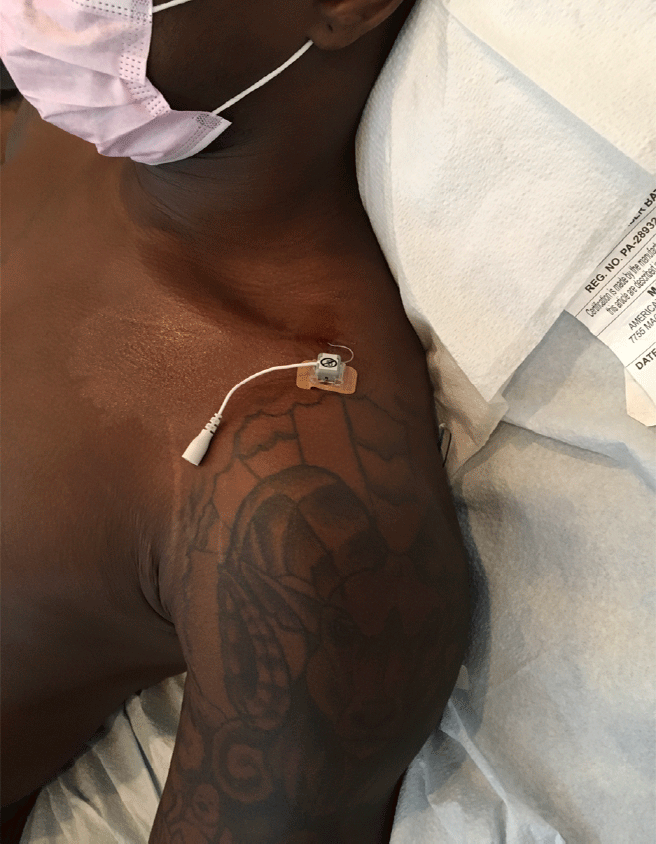
Figure 3: A brachial plexus 60 day PNS implant placed for chronic postoperative pain control after a work related hand injury

Figure 4: A suprascapular permanent PNS placed for chronic postoperative pain following total shoulder replacement

Figure 5: An Ilio-inguinal (lateral) and genito-femoral (medial) 60 -day PNS system in a patient with chronic pelvic pain; (left image) A post-operative ilio-inguinal permanent PNS showing an external pulse transmitter placed for post-herniorrhaphy pain. (right image)

Figure 6: A femoral nerve permanent PNS placed to control stump pain in an above knee amputee
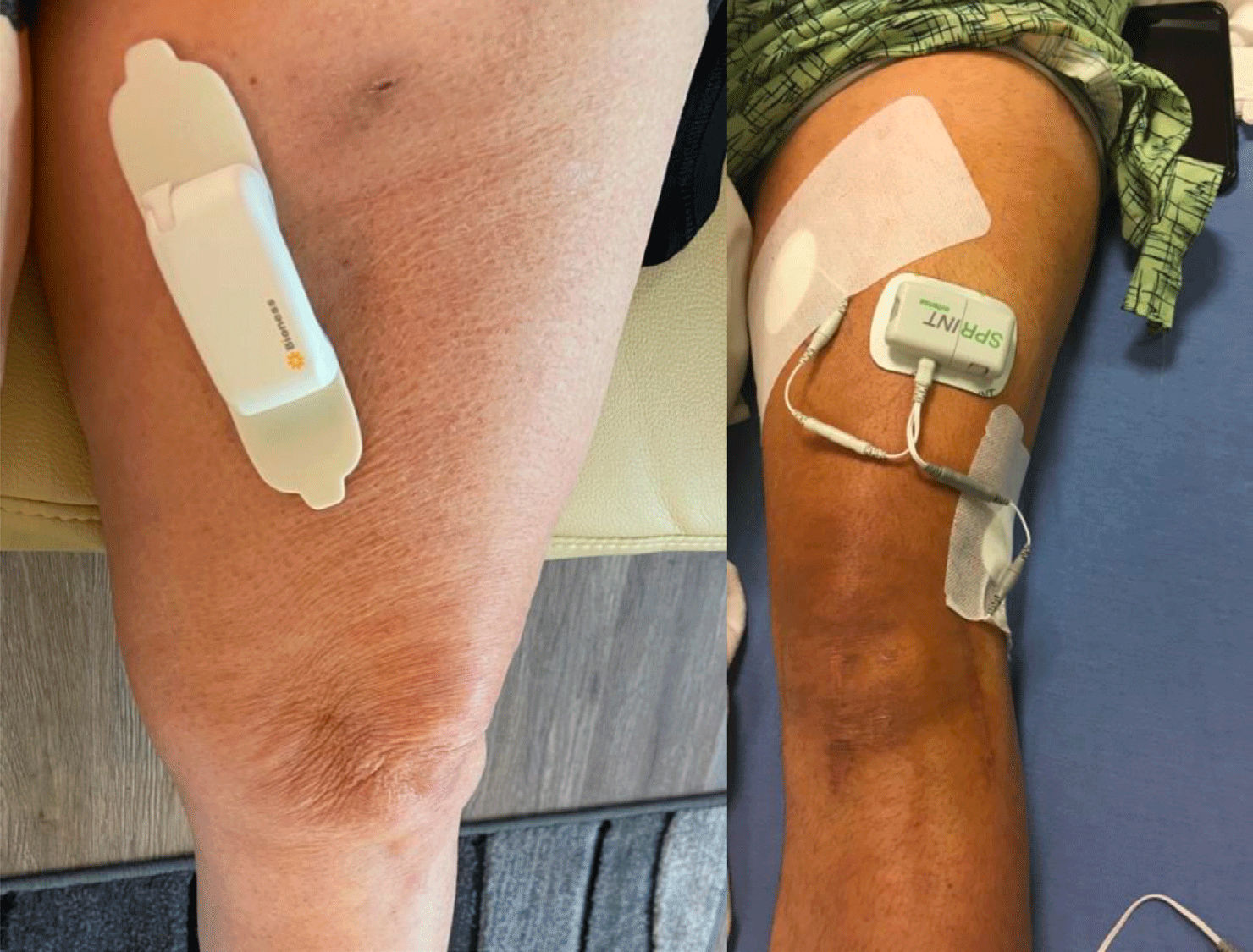
Figure 7: A saphenous permanent PNS in a patient with chronic medial ankle pain after ankle replacement and a saphenous + sciatic 60day PNS system in a young patient with chronic knee pain.
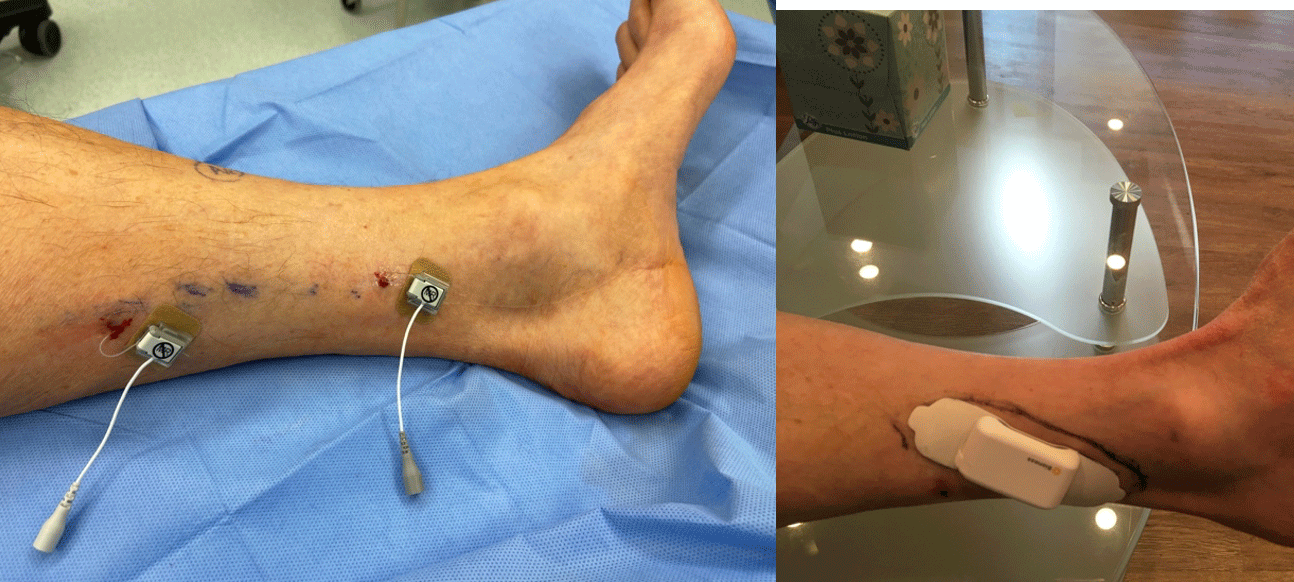
Figure 8: A tibial 60 day PNS system and tibial permanent PNS for failed tarsal tunnel release surgery
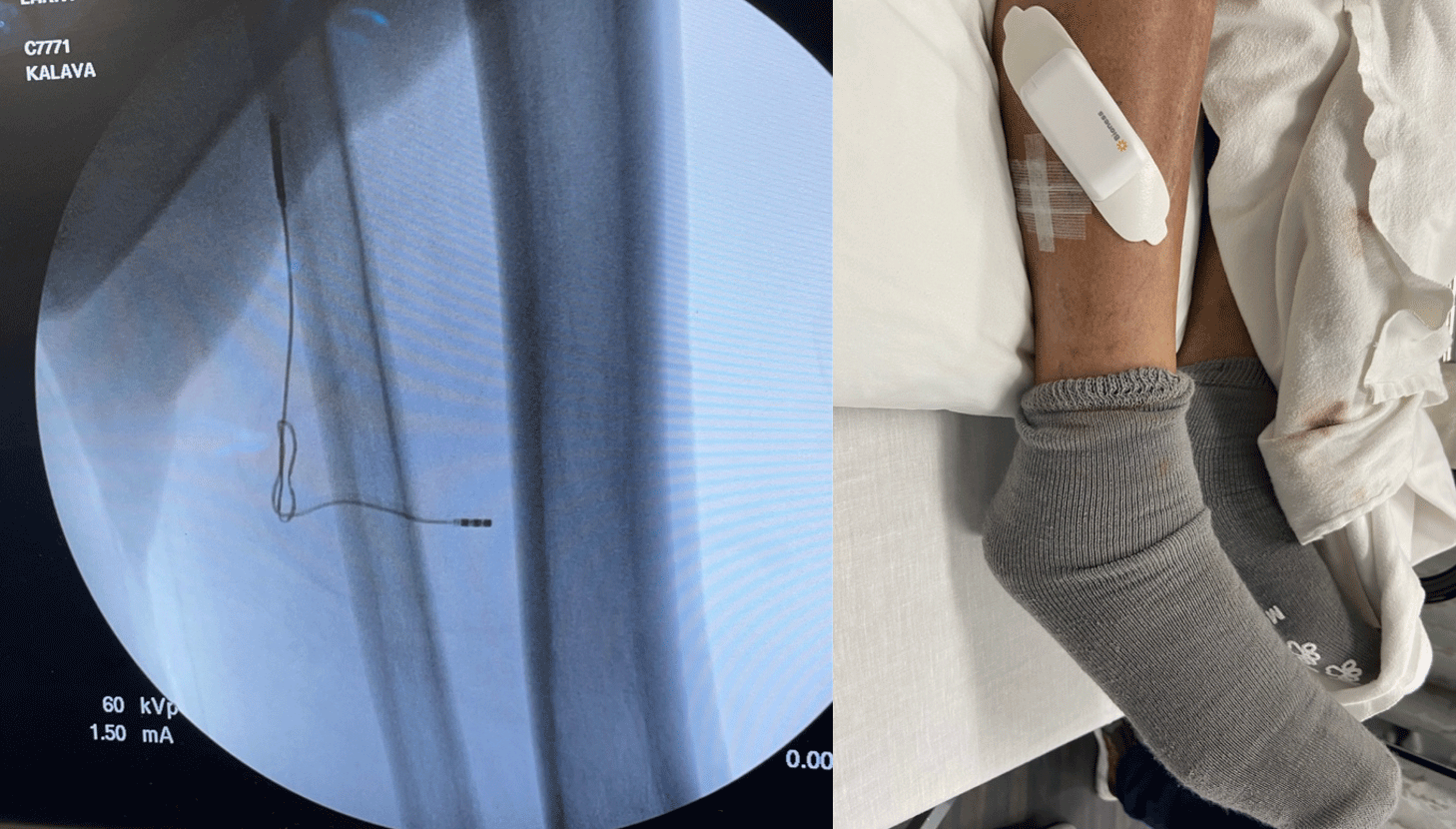
Figure 9: A superficial peroneal nerve permanent PNS for chronic post-surgical ankle pain
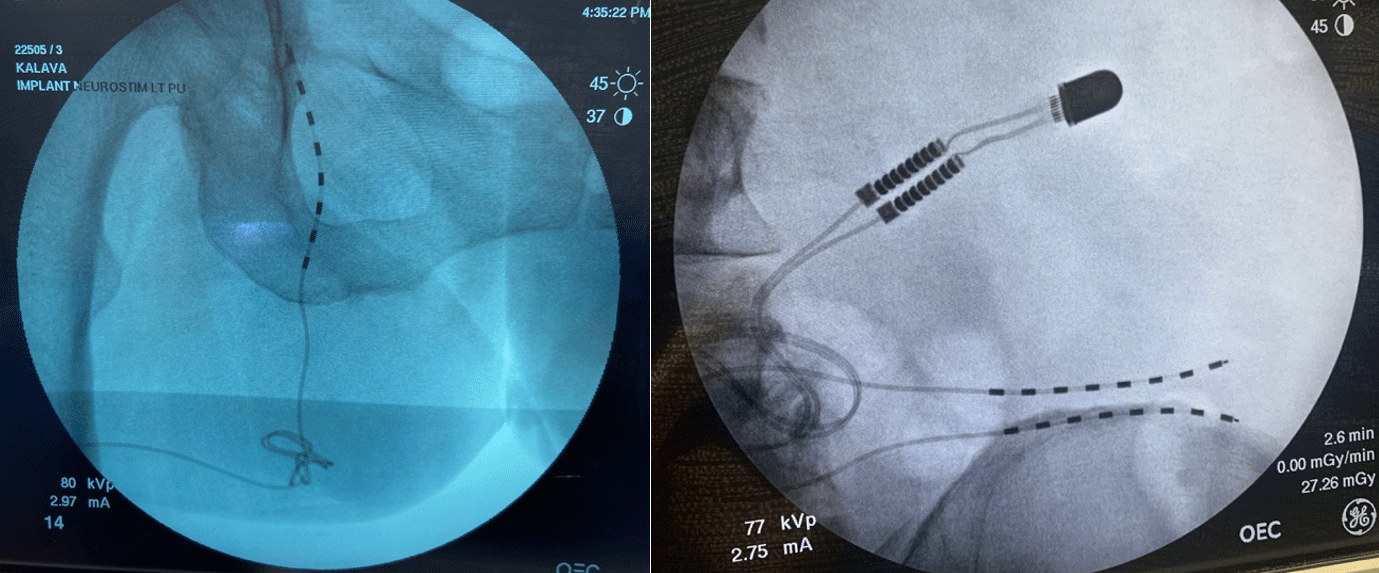
Figure 10: A pudendal permanent PNS implant for patient with chronic pelvic pain (left image); A superior cluneal nerve permanent PNS implant with small button sized internal pulse generator (IPG) for chronic low back pain from cluneal neuralgia (right image)
Table 1: Commonly performed PNS implants and the proposed regional anesthetic
| Commonly performed PNS implants | Proposed regional anesthetic |
|---|---|
| Popliteal fossa: Sciatic nerve (Figure 2) | Sciatic nerve block with mid-thigh, subgluteal, transgluteal approach or isolated tibial & common peroneal nerve blocks |
| Interscalene groove: Brachial plexus | Suprascapular and Axillary nerve block; Superior trunk block. Cervical erector spinae plane block or Cervical retrolaminar Block |
| Supraclavicular fossa: Brachial plexus (Figure 3) | Infraclavicular or Axillary brachial plexus block |
| Suprascapular notch: Suprascapular nerve (Figure 4) | Interscalene brachial plexus block/Anterior suprascapular nerve block or superior trunk block |
| Intercostal rib space: Intercostal nerve | Serratus plane block, erector spinae plane block, or paravertebral block |
| Anterior superior iliac spine: Ilioinguinal/iliohypogastric nerve (Figure 5) | T12/L1 paravertebral block or transversus abdominus plane block or quadratus lumborum block |
| Femoral triangle: Femoral nerve (Figure 6) | Adductor canal block or lumbar plexus block or pericapsular nerve group (PENG) block |
| Adductor canal: Saphenous nerve (Figure 7) | Femoral nerve block, lumbar plexus block or saphenous nerve block below the knee or femoral triangle block |
| Medial ankle: Tibial nerve (Figure 8) | Isolated tibial nerve block at the popliteal fossa |
| Calf: Sural nerve | Sural nerve block at the ankle or sciatic nerve block at the popliteal fossa |
| Lateral leg: Superficial peroneal nerve (Figure 9) | Superficial peroneal nerve block at the ankle or common peroneal nerve block at the popliteal fossa |
| Pelvis: Pudendal nerve & Superior cluneal nerves (Figure 10) | Pudendal nerve block in lithotomy position; For superior cluneal nerves, consider T12-L3 paravertebral nerve blocks or lumbar erector spinae plane block |
“To get Game-Changing results, start focusing on Game-Changing thoughts.”



